Quantum Mechanical Model of Atom
Quantum Mechanical Model of Atom: Overview
This topic covers concepts, such as, Quantum Mechanical Model of Atom, Schrodinger's Wave Equation, Angular Nodes & Nodes or Nodal Surfaces etc.
Important Questions on Quantum Mechanical Model of Atom
The number of spherical nodes in 3p orbitals are
Which orbital among the following has zero radial nodes and angular nodes?
The number of radial nodes in and orbitals are respectively:
Choose the correct statement among the following:
The number of nodes in the orbital is _____
Which statement(s) concerning Bohr's model is incorrect?
For next two question please follow the same
The hydrogen - like species Li2+ is in a spherically symmetric state S1 with one radial node. Upon absorbing light, the ion undergoes transition to a state S2. The state S2 has one radial node and its energy is equal to the ground state energy of the hydrogen atom.
The energy of the S1 in units of the hydrogen atom ground state energy is
The number of nodal planes ‘ ’ orbital has, is:
The orbitals which have same number of nodes are
| Column I (orbital) | Column II (Radial nodes) | ||
| A | 3p | p | 0 |
| B | 5d | q | 1 |
| C | 4f | r | 2 |
| D | 4s | s | 3 |
For an electron in a H-atom, find out the exact ratio of probability of finding the electron at the nucleus to the probability of finding it at Bohr radius .
The wave function is .
The wave function of an atomic orbital is given as . What is the correct statement regarding this orbital?
How does the probability distribution curve for 2s electron appears?
(Assume that all graphs have been plotted for the same parameters. In all these curves n )
Choose the correct radial probability curve for orbital.
What is the total number of all types of nodes possible in the highest energy orbital of the third shell in a multi electron atom?
The hydrogen-like species is with one radial node in a spherically symmetric state . On absorbing the light ion will undergo a transition from state to a state . The state has one radial node and its energy is equal to the ground state energy of the hydrogen atom.
The state will be
According to the modern atomic theory, calculate the angular momentum, spherical nodes and angular nodes of 210 respectively?
Number of radial nodes for orbital
From the following graph between R (radial wave function) and r (distance from nucleus), identify orbital:-
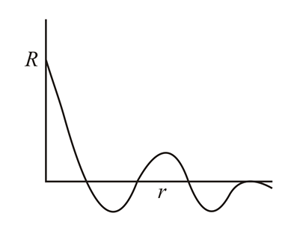
The d-orbital with two nodal cones:
